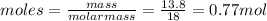Chemistry, 14.07.2019 04:30 parisaidan366
How much heat is absorbed to change 13.8 grams of h2o from a solid to a liquid at zero degrees celsius? δhfusion = 6.03 kj/mol δhvaporization = 40.65 kj/mol 31.1 kj 561 kj 4.62 kj 83.2 kj

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

You know the right answer?
How much heat is absorbed to change 13.8 grams of h2o from a solid to a liquid at zero degrees celsi...
Questions



Health, 13.04.2021 06:40



Social Studies, 13.04.2021 06:40

Mathematics, 13.04.2021 06:40


Biology, 13.04.2021 06:40


Mathematics, 13.04.2021 06:40

Chemistry, 13.04.2021 06:40

Biology, 13.04.2021 06:40

Mathematics, 13.04.2021 06:40




English, 13.04.2021 06:40