

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1
You know the right answer?
Chem ! using the average atomic mass, calculate the number of atoms present in 1.2 ml of liquid mer...
Questions

Mathematics, 14.07.2019 06:30

History, 14.07.2019 06:30


Biology, 14.07.2019 06:30




English, 14.07.2019 06:30


Chemistry, 14.07.2019 06:30


Biology, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30



History, 14.07.2019 06:30

Chemistry, 14.07.2019 06:30



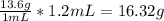
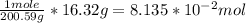
![\frac{6.022*10^{23}atoms}{1mole} *[8.135*10^{-2}mol]=4.90*10^{22}atoms](/tpl/images/0087/4847/f8efe.png)
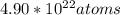 present.
present.

