
Chemistry, 14.07.2019 06:00 achoward08
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reaction is at dynamic equilibrium, at what rate will substance b be consumed? 0.0 mol/l·s 1.0 mol/l·s 2.0 mol/l·s 4.0 mol/l·s

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reacti...
Questions

Mathematics, 04.01.2020 22:31

History, 04.01.2020 22:31

Social Studies, 04.01.2020 22:31



Mathematics, 04.01.2020 22:31

Mathematics, 04.01.2020 22:31

Spanish, 04.01.2020 22:31

History, 04.01.2020 22:31

Social Studies, 04.01.2020 22:31

Mathematics, 04.01.2020 22:31

History, 04.01.2020 22:31



Mathematics, 04.01.2020 22:31




Biology, 04.01.2020 22:31

Mathematics, 04.01.2020 22:31

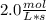
![R(a) = k * \frac{d[A]}{dt}](/tpl/images/0087/5945/2a16d.png)
![R (b)= k * \frac{d[B]}{dt}](/tpl/images/0087/5945/bbb74.png)


