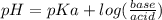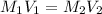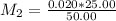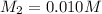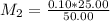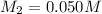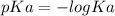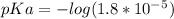Chemistry, 14.07.2019 09:00 anthonyfr10004
What is the ph of a solution prepared by mixing 25.00 ml of 0.10 m ch3co2h with 25.00 ml of 0.020 m ch3co2na? assume that the volume of the solutions are additive and that k a = 1.8 × 10-5 for ch3co2h?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 1

Chemistry, 23.06.2019 17:00
Identify the missing coefficient in the balanced equation and classify the type of reaction. mg(oh)2 + h2so4 ⟶ mgso4 + 1; combustion 1; neutralization 2; combustion 2; neutralization
Answers: 1

Chemistry, 23.06.2019 20:20
Identify the branch of chemistry. percentage purity of glucose.
Answers: 2
You know the right answer?
What is the ph of a solution prepared by mixing 25.00 ml of 0.10 m ch3co2h with 25.00 ml of 0.020 m...
Questions

Health, 02.07.2021 01:00










Mathematics, 02.07.2021 01:00



Mathematics, 02.07.2021 01:00

Mathematics, 02.07.2021 01:00