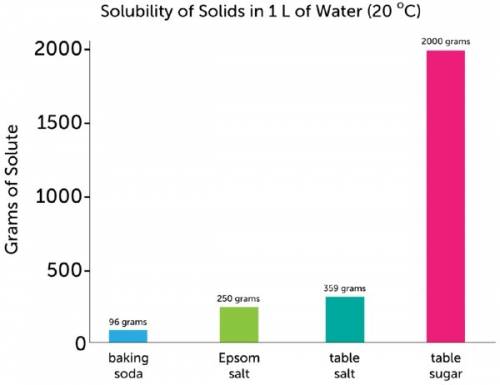
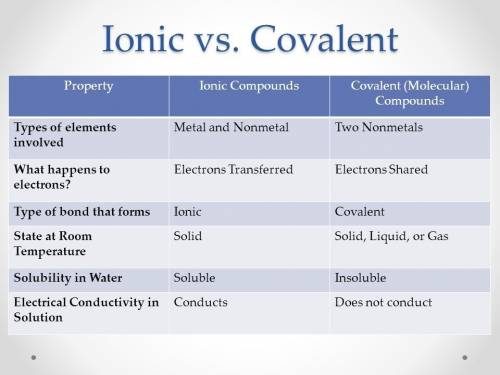
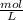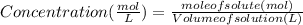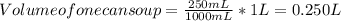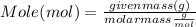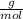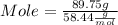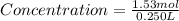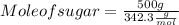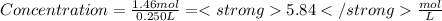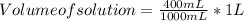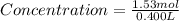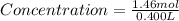Chemistry, 30.09.2019 08:20 chloemandile9818
Maybe someone can me out with this or give me the equation to find mols/liters - to if you can , here's the question given to me.
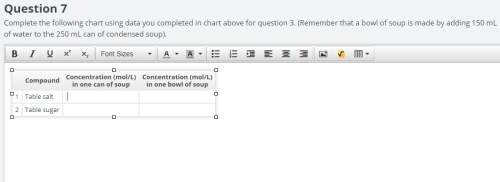
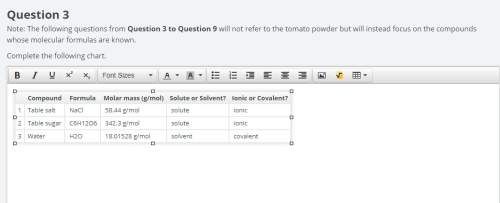
complete the following chart using data you completed in chart above for question 3. (remember that a bowl of soup is made by adding 150 ml of water to the 250 ml can of condensed soup).
the chart asks for the concentration (mol/l)
in one can of soup concentration (mol/l)
in one bowl of soup
-both of them are asking for table salt and table sugar


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
Maybe someone can me out with this or give me the equation to find mols/liters - to if you can , h...
Questions

English, 02.10.2021 15:20

Mathematics, 02.10.2021 15:20


Chemistry, 02.10.2021 15:20





English, 02.10.2021 15:20

Mathematics, 02.10.2021 15:20



Biology, 02.10.2021 15:20

Mathematics, 02.10.2021 15:20

Mathematics, 02.10.2021 15:20


Chemistry, 02.10.2021 15:20


Mathematics, 02.10.2021 15:20