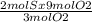Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
Consider the reaction between reactants s and o2: 2s(s)+3o2(g)→2so3(g)if a reaction vessel initiall...
Questions


Mathematics, 08.12.2021 03:30

Mathematics, 08.12.2021 03:30

Arts, 08.12.2021 03:30


Arts, 08.12.2021 03:30

Mathematics, 08.12.2021 03:30

Physics, 08.12.2021 03:30


Mathematics, 08.12.2021 03:30



English, 08.12.2021 03:30

Chemistry, 08.12.2021 03:30

Mathematics, 08.12.2021 03:30


Social Studies, 08.12.2021 03:30

Geography, 08.12.2021 03:30


Mathematics, 08.12.2021 03:30