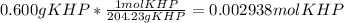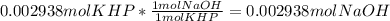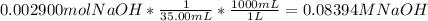Chemistry, 14.07.2019 12:00 potatogirl6811
Reagent grade potassium hydrogen phthalate (khp, mass 204.23g/mole) is a high molecular weight, stable, monoprotic solid acid. it is commonly used for standardizing sodium hydroxide solutions. what concentration of sodium hydroxide solution would be needed to titration 0.6000g of khp so that the volume of naoh needed is 35.00ml

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
Reagent grade potassium hydrogen phthalate (khp, mass 204.23g/mole) is a high molecular weight, stab...
Questions

Biology, 27.03.2020 22:17


Mathematics, 27.03.2020 22:18






Health, 27.03.2020 22:18

History, 27.03.2020 22:18



Mathematics, 27.03.2020 22:18


English, 27.03.2020 22:18

Computers and Technology, 27.03.2020 22:18

Chemistry, 27.03.2020 22:18