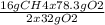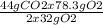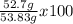Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 and 78.3g o2 to react. when the reaction is finished, the chemist collects 52.7g co2. determine the limiting reagent, theoretical yield, and percent yield for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 an...
Questions

Biology, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Social Studies, 17.09.2020 22:01

Physics, 17.09.2020 22:01

English, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

World Languages, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

English, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01