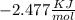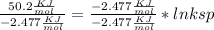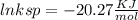Chemistry, 14.07.2019 13:30 Yorlin4441
Use the thermodynamic data at 298 k below to determine the ksp for barium carbonate, baco3 at this temperature. substance: ba2+(aq) co32–(aq) baco3(s) δh°f (kj/mol): –538.36 –676.26 –1219 δg°f (kj/mol): –560.7 –528.1 –1139 s°(j/k·mol): 13 –53.1 112

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Use the thermodynamic data at 298 k below to determine the ksp for barium carbonate, baco3 at this t...
Questions









Mathematics, 19.05.2021 04:20



Mathematics, 19.05.2021 04:20

English, 19.05.2021 04:20


Biology, 19.05.2021 04:20


Mathematics, 19.05.2021 04:20


Health, 19.05.2021 04:20

Mathematics, 19.05.2021 04:20

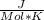
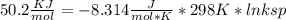
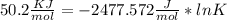
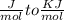
 ))
))