Chemistry, 14.07.2019 13:30 meganjackson155
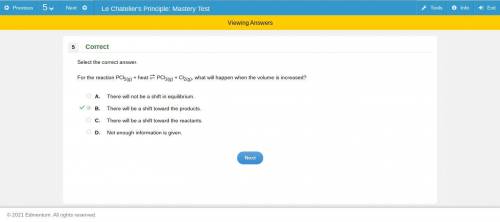
For the reaction pcl5(g) + heat pcl3(g) + cl2(g), what will happen when the volume is increased? a. there will not be a shift in equilibrium. b. there will be a shift toward the products. c. there will be a shift toward the reactants. d. not enough information is given. if you can figure out the answer, you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1


Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
For the reaction pcl5(g) + heat pcl3(g) + cl2(g), what will happen when the volume is increased? a....
Questions

Mathematics, 02.09.2020 01:01

Mathematics, 02.09.2020 01:01

Mathematics, 02.09.2020 01:01


Computers and Technology, 02.09.2020 01:01

Mathematics, 02.09.2020 01:01






Biology, 02.09.2020 01:01


Spanish, 02.09.2020 01:01


Mathematics, 02.09.2020 01:01

Chemistry, 02.09.2020 01:01


Mathematics, 02.09.2020 01:01