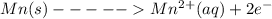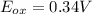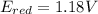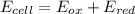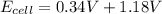Chemistry, 14.07.2019 14:30 sciencecreation87
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq) the reduction half-reaction has a standard potential of 1.18 v. the oxidation half-reaction has a standard potential of 0.34 v. what is the overall cell potential? (a) -1.52 v (b) -1.18 v (c) +1.18 v (d) +1.52 v

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq)...
Questions

Mathematics, 11.01.2021 19:00



English, 11.01.2021 19:00





Mathematics, 11.01.2021 19:00


English, 11.01.2021 19:00


Chemistry, 11.01.2021 19:00

Mathematics, 11.01.2021 19:00

Mathematics, 11.01.2021 19:00


Mathematics, 11.01.2021 19:00

Biology, 11.01.2021 19:00

Social Studies, 11.01.2021 19:00

English, 11.01.2021 19:00