Chemistry, 14.07.2019 15:00 Nickanderson21
According to the first law of thermodynamics, the amount of work done by a heat engine equals the amount of a. work done on the engine. b. waste heat it produces. c. thermal energy added to the engine minus the waste heat. d. thermal energy added to the engine plus the waste heat.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2


Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
According to the first law of thermodynamics, the amount of work done by a heat engine equals the am...
Questions

Mathematics, 29.11.2020 23:40


Chemistry, 29.11.2020 23:40


Mathematics, 29.11.2020 23:40

Arts, 29.11.2020 23:40

Mathematics, 29.11.2020 23:40


Health, 29.11.2020 23:40




Biology, 29.11.2020 23:40



Biology, 29.11.2020 23:40


History, 29.11.2020 23:40


Mathematics, 29.11.2020 23:40

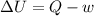
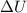 = Internal energy
= Internal energy