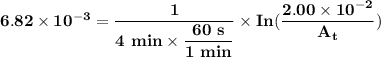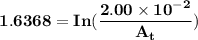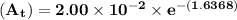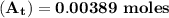Chemistry, 14.07.2019 17:30 guccikathyyy6195
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×10−3 s−1. suppose we start with 2.00×10−2 mol of n2o5(g) in a volume of 2.3 l . you may want to reference (page) section 14.4 while completing this problem. part a how many moles of n2o5 will remain after 4.0 min ? ,

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×...
Questions







French, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00

Chemistry, 21.04.2021 19:00



Biology, 21.04.2021 19:00


Mathematics, 21.04.2021 19:00


English, 21.04.2021 19:00

Mathematics, 21.04.2021 19:00

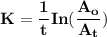
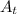 = ???
= ???