Chemistry, 14.07.2019 18:00 dootdootkazoot
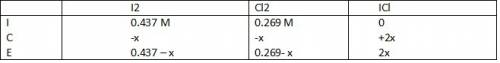
Consider the reaction between iodine gas and chlorine gas to form iodine monochloride. a reaction mixture at 298.15k initially contains i2 =0.437m and ci2=0.269m. what is concentration of ici when reaches equilibrium? keq= 81.9 @298.15k

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
Consider the reaction between iodine gas and chlorine gas to form iodine monochloride. a reaction mi...
Questions





Mathematics, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31




Mathematics, 14.01.2020 18:31


English, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

Biology, 14.01.2020 18:31



Mathematics, 14.01.2020 18:31


![K_{eq} = \frac{[ICl]^{2}}{[I_{2}][Cl_{2}]}](/tpl/images/0089/5177/ae97d.png)
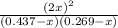
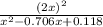
![(2x)^{2} = 81.9 [ x^{2} -0.706x + 0.118]](/tpl/images/0089/5177/63f20.png)
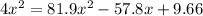
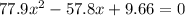
![[ICl]_{eq} = 2 ( 0.254) = 0.508 M](/tpl/images/0089/5177/bbf8d.png)