

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

You know the right answer?
Assuming the volume of the insoluble substance to be 92.5 ml and the mass to be 693.8 g, what is the...
Questions



Biology, 29.10.2021 21:20

Mathematics, 29.10.2021 21:20

Mathematics, 29.10.2021 21:20


English, 29.10.2021 21:20


Mathematics, 29.10.2021 21:20


Mathematics, 29.10.2021 21:30

Mathematics, 29.10.2021 21:30


SAT, 29.10.2021 21:30






Arts, 29.10.2021 21:30

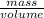
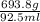 = 7.50 g/ml
= 7.50 g/ml

