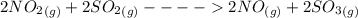Sulfuric acid was once produced through the reaction of sulfur trioxide with water. sulfur trioxide can form through the reaction of sulfur dioxide and oxygen gas. when nitrogen monoxide gas is added to the system, the reaction speeds up significantly because it proceeds through the following steps: 2no(g)+o2(> 2no2(g) 2no2(g)+> 2no(g)+2so3(g) identify the catalyst in this reaction, explain how you know it is the catalyst, and describe how it increases the rate of the reaction.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2
You know the right answer?
Sulfuric acid was once produced through the reaction of sulfur trioxide with water. sulfur trioxide...
Questions

Spanish, 07.12.2020 20:00

Social Studies, 07.12.2020 20:00

Arts, 07.12.2020 20:00



Mathematics, 07.12.2020 20:00


History, 07.12.2020 20:00

Chemistry, 07.12.2020 20:00




Biology, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00

Computers and Technology, 07.12.2020 20:00

Social Studies, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00