

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb2+ + 2cl -, the concentration of the prod...
Questions





English, 31.10.2021 07:50



Mathematics, 31.10.2021 07:50

Mathematics, 31.10.2021 07:50

Biology, 31.10.2021 07:50




Mathematics, 31.10.2021 07:50

English, 31.10.2021 07:50

English, 31.10.2021 08:00

English, 31.10.2021 08:00

Mathematics, 31.10.2021 08:00

Mathematics, 31.10.2021 08:00

Physics, 31.10.2021 08:00

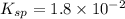
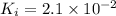
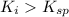 Precipitation
Precipitation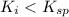 No Precipitation
No Precipitation

