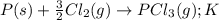Chemistry, 15.07.2019 13:00 pablogonzaleztellez
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction in terms of the equilibrium constants, ka and kb, for reactions a and b below: a.)p(s) + 5/2 cl2( g. pcl5( g. ka b.)pcl3( g. + cl2( g. pcl5( g. kb

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

You know the right answer?
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction...
Questions


Chemistry, 24.02.2021 19:00




Mathematics, 24.02.2021 19:10


Mathematics, 24.02.2021 19:10

Mathematics, 24.02.2021 19:10

History, 24.02.2021 19:10



World Languages, 24.02.2021 19:10


Mathematics, 24.02.2021 19:10

Social Studies, 24.02.2021 19:10

Mathematics, 24.02.2021 19:10



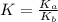
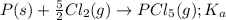
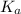 for the above equation is:
for the above equation is:![K_a=\frac{[PCl_5]}{[P][Cl_2]^{5/2}}](/tpl/images/0092/5571/bd7b7.png) ......(1)
......(1)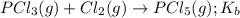
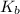 for the above equation is:
for the above equation is:![K_b=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0092/5571/bd7b0.png) ......(2)
......(2)![\frac{K_a}{K_b}=\left(\frac{\frac{[PCl_5]}{[P][Cl_2]^{5/2}}}{\frac{[PCl_5]}{[PCl_3][Cl_2]}}\right)\\\\\\\frac{K_a}{K_b}=\frac{[PCl_3]}{[P][Cl_2]^{3/2}}](/tpl/images/0092/5571/fcd9a.png)