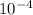Chemistry, 15.07.2019 19:00 lerasteidl
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) determine the direction of reaction if 1.00 m nocl is combined with 0.500 m no and 0.500 m cl2 in a sealed flask. a.)no reaction occurs. b.)reaction proceeds to the right toward the products. c.)reaction proceeds to the left toward the reactants. d.)reaction proceeds to the left toward the products.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) deter...
Questions

English, 28.07.2019 11:00




English, 28.07.2019 11:00


Social Studies, 28.07.2019 11:00


Business, 28.07.2019 11:00



Biology, 28.07.2019 11:00

Biology, 28.07.2019 11:00

Biology, 28.07.2019 11:00



History, 28.07.2019 11:00


Biology, 28.07.2019 11:00

Social Studies, 28.07.2019 11:00

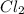
![Q = \frac{[ NO]^{2}. [Cl_{2} ]}{[ NOCl ]^{2}}](/tpl/images/1058/5527/aa67c.png)