
Chemistry, 15.07.2019 21:30 paralaw61772
Calculate the mass of hcl required to prepare 2.5 liters of a 0.08 molar solution of hcl. 1 h 1.01 hydrogen 17 cl 35.45 chlorine a. 3.2 grams b. 4.5 grams c. 7.3 grams d. 11.4 grams

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Calculate the mass of hcl required to prepare 2.5 liters of a 0.08 molar solution of hcl. 1 h 1.01 h...
Questions

Social Studies, 19.09.2020 01:01

Biology, 19.09.2020 01:01



Biology, 19.09.2020 01:01















Mathematics, 19.09.2020 01:01

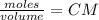
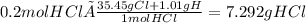 it's the choice c
it's the choice c

