
Chemistry, 15.07.2019 23:30 devonyam7965
Asample of phosgene gas at an initial concentration of 0.500 m is heated at 527 °c in a reaction vessel. at equilibrium, the concentration of co (g) was found to be 0.046 m. calculate the equilibrium constant for the reaction at 527 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
You know the right answer?
Asample of phosgene gas at an initial concentration of 0.500 m is heated at 527 °c in a reaction ves...
Questions






Mathematics, 09.09.2020 20:01

Mathematics, 09.09.2020 20:01



Biology, 09.09.2020 20:01







Mathematics, 09.09.2020 20:01


English, 09.09.2020 20:01

Business, 09.09.2020 20:01

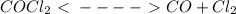
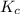 ;
;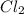 ] /
] / ![[COCl_{2} ]](/tpl/images/0094/2379/d6274.png)


