
Chemistry, 16.07.2019 04:00 cmfield8810
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain why the shift occurs. b. what would have been observed if hcl (also a strong acid) had been added instead of the hno3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain w...
Questions






Mathematics, 17.10.2020 15:01



Mathematics, 17.10.2020 15:01





Mathematics, 17.10.2020 15:01



History, 17.10.2020 15:01

Mathematics, 17.10.2020 15:01


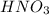 reacts with
reacts with 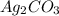 usually the equilibrium shifts to the right because
usually the equilibrium shifts to the right because 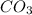 molecule from the reaction mixture, which then tends to shift the equilibrium towards right.
molecule from the reaction mixture, which then tends to shift the equilibrium towards right.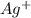 and
and 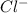 ions from the solution.
ions from the solution.

