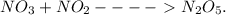Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
Dinitrogen pentoxide gas is obtained by the reaction of nitrogen trioxide gas and nitrogen dioxide g...
Questions





History, 06.10.2019 17:30

Biology, 06.10.2019 17:30

History, 06.10.2019 17:30

Mathematics, 06.10.2019 17:30

Chemistry, 06.10.2019 17:30




History, 06.10.2019 17:30


Health, 06.10.2019 17:30

Mathematics, 06.10.2019 17:30

Chemistry, 06.10.2019 17:30



 .
.