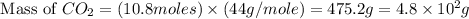Consider the reaction between acetylene, c2h2, and oxygen in a welding torch: 2c2h2(g) + 5o2(g) → 4co2(g) + 2h2o(g) if 5.4 moles of acetylene react with sufficient oxygen, how many grams of co2 will be formed? a. 2.4 × 102 g b. 9.5 × 102 g c. 4.8 × 102 g d. 1.5 × 102 g e. 0.49 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Consider the reaction between acetylene, c2h2, and oxygen in a welding torch: 2c2h2(g) + 5o2(g) → 4...
Questions

English, 20.08.2021 09:10

Biology, 20.08.2021 09:10

Mathematics, 20.08.2021 09:20

History, 20.08.2021 09:20

Computers and Technology, 20.08.2021 09:20

Computers and Technology, 20.08.2021 09:20

Mathematics, 20.08.2021 09:20



Social Studies, 20.08.2021 09:20



Mathematics, 20.08.2021 09:20

Social Studies, 20.08.2021 09:20

Business, 20.08.2021 09:20


English, 20.08.2021 09:20

Mathematics, 20.08.2021 09:20

English, 20.08.2021 09:20

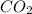 formed will be,
formed will be, 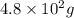
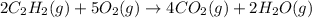
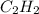 react to give 4 mole of
react to give 4 mole of 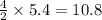 moles of
moles of