
Chemistry, 16.07.2019 09:30 manarsadi6
Achemical engineer determines the mass percent of iron in an ore sample by converting the fe to fe2+ in acid and then titrating the fe2+ with mno4−. a 1.3909−g sample was dissolved in acid and then titrated with 21.63 ml of 0.04462 m kmno4. the balanced equation is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

You know the right answer?
Achemical engineer determines the mass percent of iron in an ore sample by converting the fe to fe2+...
Questions






English, 10.02.2021 18:50

Mathematics, 10.02.2021 18:50


Mathematics, 10.02.2021 18:50






Mathematics, 10.02.2021 18:50



Mathematics, 10.02.2021 18:50


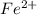 in acid and then titrating the
in acid and then titrating the 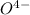 .
. 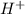 (aq) + 5
(aq) + 5 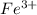 (aq) +
(aq) + 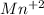 +(aq) + 4
+(aq) + 4 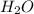 (l)
(l) 

