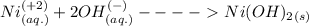Chemistry, 16.07.2019 17:00 dollangellface22
Consider the reaction when aqueous solutions of nickel(ii) nitrate and potassium hydroxide are combined. (use the solubility rules provided in the owl preparation page to determine the solubility of compounds.) the net ionic equation for this reaction is:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Consider the reaction when aqueous solutions of nickel(ii) nitrate and potassium hydroxide are combi...
Questions

Computers and Technology, 22.07.2019 02:30


Biology, 22.07.2019 02:30

Biology, 22.07.2019 02:30

Mathematics, 22.07.2019 02:30

English, 22.07.2019 02:30

History, 22.07.2019 02:30

Mathematics, 22.07.2019 02:30


Mathematics, 22.07.2019 02:30

Health, 22.07.2019 02:30




English, 22.07.2019 02:30





History, 22.07.2019 02:30

![Ni(NO _{3}) _{2} + 2 KOH ----\ \textgreater \ Ni(OH) _{2} + 2 [KNO _{3} ].](/tpl/images/0097/0401/ab3f8.png)