Chemistry, 17.07.2019 08:00 crawford184232323234
What is the value of the equilibrium constant for this redox reaction? 2ag+(aq) + zn (s) → 2ag (s) + zn2+(aq) e = +1.56 v k=10(ne∘0.0592) k = 2.25 × 10-26 k = 2.25 × 1026 k = 5.04 × 10-52 k = 5.04 × 1052

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

You know the right answer?
What is the value of the equilibrium constant for this redox reaction? 2ag+(aq) + zn (s) → 2ag (s)...
Questions


English, 24.03.2021 21:20


Mathematics, 24.03.2021 21:20

Mathematics, 24.03.2021 21:20

History, 24.03.2021 21:20

Computers and Technology, 24.03.2021 21:20

Mathematics, 24.03.2021 21:20



Mathematics, 24.03.2021 21:20

Business, 24.03.2021 21:20


Mathematics, 24.03.2021 21:20

English, 24.03.2021 21:20





Mathematics, 24.03.2021 21:20

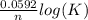 ,
,