
Chemistry, 09.10.2019 19:30 estefaniapenalo
From the diagram, when the temperatures of reactants are raised from 20°c to 30°c:
a. the average kinetic energy doubles.
b. the number of molecules doubles.
c. the total number of collisions doubles.
d. the number of molecules having er or greater doubles.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
From the diagram, when the temperatures of reactants are raised from 20°c to 30°c:
a. the ave...
a. the ave...
Questions

History, 27.07.2019 02:10

History, 27.07.2019 02:10

Mathematics, 27.07.2019 02:10

Mathematics, 27.07.2019 02:10


English, 27.07.2019 02:10

Mathematics, 27.07.2019 02:10













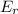 which means now double number of molecules will be able to cross the energy barrier and hence the formation of product increases and hence the rate increases to double.
which means now double number of molecules will be able to cross the energy barrier and hence the formation of product increases and hence the rate increases to double.

