
Chemistry, 17.07.2019 15:30 susanasalleyp3enwe
Weight of one mole of carbon = 12.01 g weight of one mole of oxygen = 16.00 g the molecular weight (gram formula weight) for co is

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
Weight of one mole of carbon = 12.01 g weight of one mole of oxygen = 16.00 g the molecular weight (...
Questions

Mathematics, 22.05.2020 22:02





Mathematics, 22.05.2020 22:02

Mathematics, 22.05.2020 22:02



Mathematics, 22.05.2020 22:02





Biology, 22.05.2020 22:02

Mathematics, 22.05.2020 22:02


Mathematics, 22.05.2020 22:02


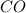 is, 28.01 g/mole
is, 28.01 g/mole

