
Chemistry, 17.07.2019 20:00 kyllow5644
Acompound has a percent composition of 40.0% carbon, 6.72% hydrogen and 53.28% oxygen. if its molar mass is 180 g/mol, what is its molecular formula?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Acompound has a percent composition of 40.0% carbon, 6.72% hydrogen and 53.28% oxygen. if its molar...
Questions




Mathematics, 27.07.2019 21:40


Mathematics, 27.07.2019 21:40

History, 27.07.2019 21:40

Biology, 27.07.2019 21:40

Social Studies, 27.07.2019 21:40

Computers and Technology, 27.07.2019 21:40



Mathematics, 27.07.2019 21:40


Mathematics, 27.07.2019 21:40


Social Studies, 27.07.2019 21:40



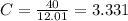
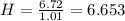
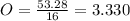
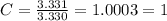
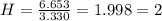
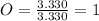
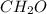
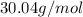
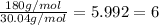
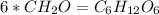
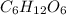 .
.

