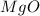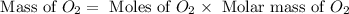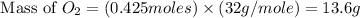Chemistry, 19.11.2019 19:31 ladypink94
Asample of 1.6 g of methane (ch4) is completely burnt in 20.00 g of oxygen. the products are carbon dioxide and water. which is the excess reactant? which is the limiting reactant? how much of the excess reactant remains unreacted?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Asample of 1.6 g of methane (ch4) is completely burnt in 20.00 g of oxygen. the products are carbon...
Questions

Mathematics, 18.08.2019 21:20

English, 18.08.2019 21:20

History, 18.08.2019 21:20



Geography, 18.08.2019 21:20

Physics, 18.08.2019 21:20


History, 18.08.2019 21:20

Mathematics, 18.08.2019 21:20

Physics, 18.08.2019 21:20

Mathematics, 18.08.2019 21:20

Social Studies, 18.08.2019 21:20



Mathematics, 18.08.2019 21:20


Computers and Technology, 18.08.2019 21:20


Chemistry, 18.08.2019 21:20

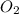
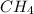
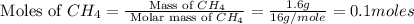
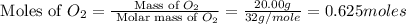
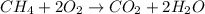
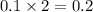 moles of
moles of