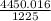Chemistry, 18.07.2019 09:30 valdiviaricky1355
Asample of ammonia gas at 75°c and 445 mm hg has a volume of 16.0 l. what volume will it occupy if the pressure rises to 1225 mm hg at constant temperature? 1 atm = 760 mm hg.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Asample of ammonia gas at 75°c and 445 mm hg has a volume of 16.0 l. what volume will it occupy if t...
Questions


Mathematics, 23.02.2020 04:22

History, 23.02.2020 04:22







English, 23.02.2020 04:24

Mathematics, 23.02.2020 04:24

Mathematics, 23.02.2020 04:24

Mathematics, 23.02.2020 04:24


Mathematics, 23.02.2020 04:24


Mathematics, 23.02.2020 04:24

Mathematics, 23.02.2020 04:25

Arts, 23.02.2020 04:25

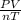 = constant
= constant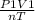 =
= 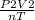
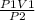 =
=