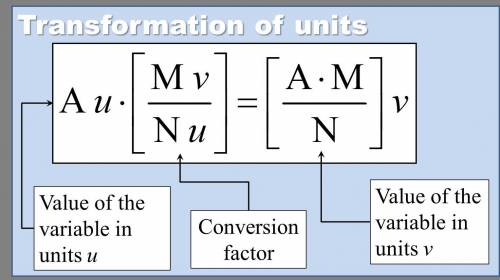
Chemistry, 18.07.2019 13:00 emilypzamora11
The pressure in car tires is often measured in pounds per square inch (lb/in.2), with the recommended pressure being in the range of 25 to 45 lb/in.2. suppose a tire has a pressure of 34.5 lb/in.2 . convert 34.5 lb/in.2 to its equivalent in atmospheres. express the pressure numerically in atmospheres.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Acompound is found to contain 6.1% hydrogen and 93.9% oxygen. find it’s empirical formula.
Answers: 2

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
The pressure in car tires is often measured in pounds per square inch (lb/in.2), with the recommende...
Questions

English, 17.09.2019 07:00



Mathematics, 17.09.2019 07:00

Geography, 17.09.2019 07:00

Mathematics, 17.09.2019 07:00

English, 17.09.2019 07:00


History, 17.09.2019 07:00

Computers and Technology, 17.09.2019 07:00



English, 17.09.2019 07:00



Business, 17.09.2019 07:00


Mathematics, 17.09.2019 07:00

Social Studies, 17.09.2019 07:00

History, 17.09.2019 07:00