
Chemistry, 19.07.2019 06:00 Jaedenaleinson
Calculate the freezing point of a solution made from 52.6 g of propane, c3h8, dissolved in 196.0 g of benzene, c6h6. the freezing point of benzene is 5.50 c and its kf is 5.12 c/m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Calculate the freezing point of a solution made from 52.6 g of propane, c3h8, dissolved in 196.0 g o...
Questions

Social Studies, 27.05.2021 17:20








English, 27.05.2021 17:20


History, 27.05.2021 17:20

Mathematics, 27.05.2021 17:20

Chemistry, 27.05.2021 17:20

Mathematics, 27.05.2021 17:20



English, 27.05.2021 17:20



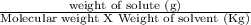
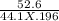 = 6.085 m
= 6.085 m

