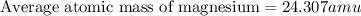Chemistry, 19.07.2019 19:00 erikagibson3414
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magnesium-24 has an abundance of 78.994% and a mass of 23.985 amu. magnesium-25 has an abundance of 10.001% and a mass of 24.986 amu. magnesium-26 has an abundance of 11.013% and a mass of 25.983 amu. calculate the average atomic mass of magnesium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magn...
Questions



Mathematics, 16.12.2020 23:00

Mathematics, 16.12.2020 23:00


Mathematics, 16.12.2020 23:00


Chemistry, 16.12.2020 23:00



English, 16.12.2020 23:00

Mathematics, 16.12.2020 23:00






Social Studies, 16.12.2020 23:00

Mathematics, 16.12.2020 23:00

Biology, 16.12.2020 23:00

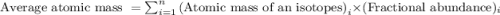 .....(1)
.....(1)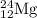 isotope:
isotope: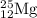 isotope:
isotope: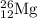 isotope:
isotope:![\text{Average atomic mass of magnesium}=[(23.985\times 0.78994)+(24.986\times 0.10001)+(25.983\times 0.11013)]](/tpl/images/0108/8899/cfd57.png)