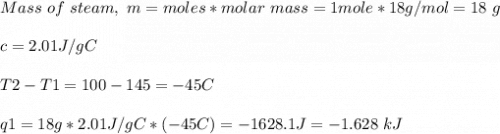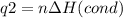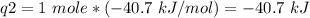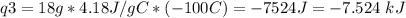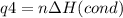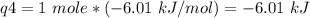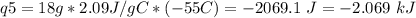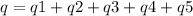Chemistry, 19.07.2019 21:00 wendelkristen
How much heat is evolved in converting 1.00 mol of steam at 145.0 ∘c to ice at -55.0 ∘c? the heat capacity of steam is 2.01 j/(g⋅∘c) and of ice is 2.09 j/(g⋅∘c).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1


Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
How much heat is evolved in converting 1.00 mol of steam at 145.0 ∘c to ice at -55.0 ∘c? the heat c...
Questions






Mathematics, 13.08.2020 22:01





English, 13.08.2020 22:01