Chemistry, 20.07.2019 00:00 jeffcarpenter
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106 × 10–3 universal mass unit less than the combined mass of the particles from which it is formed. approximately how much energy is released when this nucleus is formed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106...
Questions

English, 05.05.2020 06:14


Mathematics, 05.05.2020 06:14


History, 05.05.2020 06:14

Physics, 05.05.2020 06:14

Mathematics, 05.05.2020 06:14


Mathematics, 05.05.2020 06:14

Mathematics, 05.05.2020 06:14




Mathematics, 05.05.2020 06:14

Mathematics, 05.05.2020 06:14

Mathematics, 05.05.2020 06:14

History, 05.05.2020 06:14

Biology, 05.05.2020 06:14


History, 05.05.2020 06:14

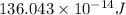
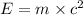
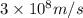
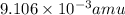
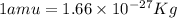
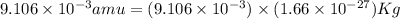
![E=[(9.106\times 10^{-3})\times (1.66\times 10^{-27})Kg]\times (3\times 10^8m/s)^2](/tpl/images/0109/7110/13e90.png)