

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
You know the right answer?
For the reaction n2 (g) + o2 (g) 2no (g), thekeq at 2130°c is 0.0025. assume [n2] = 0.95 m, [o2] =...
Questions



History, 22.09.2019 21:10

Mathematics, 22.09.2019 21:10

Mathematics, 22.09.2019 21:10

Mathematics, 22.09.2019 21:10




Mathematics, 22.09.2019 21:10







French, 22.09.2019 21:10



Arts, 22.09.2019 21:10

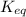
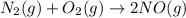
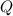 is written as:
is written as:![Q=\frac{[NO]^2}{[N_2]^1[O_2]^1}](/tpl/images/0110/0991/f0107.png)
![Q=\frac{[0.050]^2}{[0.95]^1[0.65]^1}](/tpl/images/0110/0991/2138c.png)
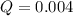
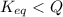 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.


