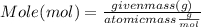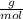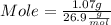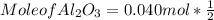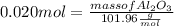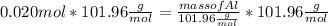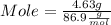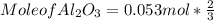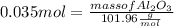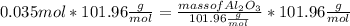Chemistry, 20.07.2019 07:30 awkwardkid0123
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g of al? 3mno2 (s) + 4al(s) -> 3mn (s) + 2al2o3 (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g...
Questions

English, 25.11.2020 07:20

Mathematics, 25.11.2020 07:20

Social Studies, 25.11.2020 07:20


History, 25.11.2020 07:20

Mathematics, 25.11.2020 07:20


Health, 25.11.2020 07:20


English, 25.11.2020 07:20

Biology, 25.11.2020 07:20

English, 25.11.2020 07:20

Mathematics, 25.11.2020 07:20

Chemistry, 25.11.2020 07:20


English, 25.11.2020 07:20

Chemistry, 25.11.2020 07:20

Mathematics, 25.11.2020 07:20

Mathematics, 25.11.2020 07:20