Chemistry, 20.07.2019 11:30 jdkrisdaimcc11
The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is a. hno3(aq) + sr(oh)2(aq) → sr(no3)2 (aq) + h2 (g) b. hno3(aq) + sr(oh)2 (aq) → h2o (l) + sr(no3)2 (aq) c. hno3 (aq) + sroh (aq) → h2o (l) + srno3(aq) d.2hno3 (aq) + sr(oh)2 (aq) → 2h2o (l) + sr(no3)2(aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 00:30
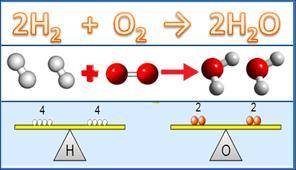
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
You know the right answer?
The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is a. hno3(aq) +...
Questions


Mathematics, 11.06.2021 17:40


Social Studies, 11.06.2021 17:40




Biology, 11.06.2021 17:40

Chemistry, 11.06.2021 17:40

Mathematics, 11.06.2021 17:40

Mathematics, 11.06.2021 17:40

Mathematics, 11.06.2021 17:40



English, 11.06.2021 17:40

Social Studies, 11.06.2021 17:40

Mathematics, 11.06.2021 17:40

Mathematics, 11.06.2021 17:40

Mathematics, 11.06.2021 17:40