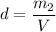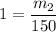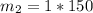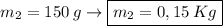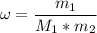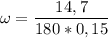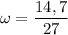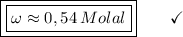Chemistry, 20.07.2019 12:00 Ruthsybel9754
What is the molality of a solution made by dissolving 14.7 g of c6h12o6 into 150.0 ml of water? assume the density of water is 1.00 g/ml?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
What is the molality of a solution made by dissolving 14.7 g of c6h12o6 into 150.0 ml of water? ass...
Questions

English, 12.10.2019 09:01



Mathematics, 12.10.2019 09:01

Mathematics, 12.10.2019 09:01

Mathematics, 12.10.2019 09:01

Mathematics, 12.10.2019 09:01

Health, 12.10.2019 09:01



Mathematics, 12.10.2019 09:01




History, 12.10.2019 09:01




History, 12.10.2019 09:01