Chemistry, 20.07.2019 13:30 Nevaeh3700
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h12o6(s) + 6o2(g) —-> 6co2(g) + 6h2o(l)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h1...
Questions

English, 17.04.2021 21:10


History, 17.04.2021 21:10


History, 17.04.2021 21:10


Mathematics, 17.04.2021 21:20

Mathematics, 17.04.2021 21:20

Mathematics, 17.04.2021 21:20









Social Studies, 17.04.2021 21:20


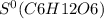 = 212.1 J/K.mol
= 212.1 J/K.mol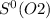 = 205.0 J/K.mol
= 205.0 J/K.mol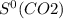 = 213.6 J/K.mol
= 213.6 J/K.mol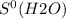 = 69.9 J/K.mol
= 69.9 J/K.mol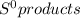 - ∑
- ∑