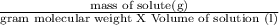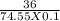Chemistry, 20.07.2019 14:30 familyk0jj3
Potassium chloride (kcl) is sometimes used to treat low blood potassium levels. calculate the concentration of a saturated solution of potassium chloride at 20°c. question continued-? hint: the formula for molarity, a common concentration unit, is m = moles/volume of solution expressed in units of mol/l. you will need to convert from grams of kcl to moles. assume the volume of solution is 100 ml. show your work! info: 36g of kcl is the max 100ml of water could hold at 20°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Potassium chloride (kcl) is sometimes used to treat low blood potassium levels. calculate the concen...
Questions


Arts, 05.03.2021 01:20

Mathematics, 05.03.2021 01:20

Mathematics, 05.03.2021 01:20



Mathematics, 05.03.2021 01:20


Biology, 05.03.2021 01:20


Mathematics, 05.03.2021 01:20

Mathematics, 05.03.2021 01:20


Social Studies, 05.03.2021 01:20

Mathematics, 05.03.2021 01:20



Mathematics, 05.03.2021 01:20

Mathematics, 05.03.2021 01:20

Mathematics, 05.03.2021 01:20