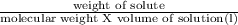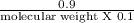Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3


Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Asolution of 0.90 g of an unknown nonelectrolyte in 100 ml of water at 27◦c has an osmotic pressure...
Questions


Advanced Placement (AP), 01.04.2020 00:21



Mathematics, 01.04.2020 00:21

English, 01.04.2020 00:21

English, 01.04.2020 00:21

History, 01.04.2020 00:21

Spanish, 01.04.2020 00:21






Mathematics, 01.04.2020 00:21

Mathematics, 01.04.2020 00:21