
Chemistry, 20.07.2019 20:00 xxYingxYangxx7311
Asolution is made by dissolving 24 g of nacl to make 475 ml of solution. calculate the concentration in units of molarity by following these steps: a) convert the grams of nacl to moles of nacl. b) calculate the liters of solution by dividing the given milliliters by 1000. c) divide moles by the liters of solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1


Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Asolution is made by dissolving 24 g of nacl to make 475 ml of solution. calculate the concentration...
Questions

Spanish, 10.11.2020 04:00

English, 10.11.2020 04:00

Computers and Technology, 10.11.2020 04:00

Mathematics, 10.11.2020 04:00




Mathematics, 10.11.2020 04:00

History, 10.11.2020 04:00

Business, 10.11.2020 04:00

Computers and Technology, 10.11.2020 04:00


Mathematics, 10.11.2020 04:00



Computers and Technology, 10.11.2020 04:00

Mathematics, 10.11.2020 04:00


Business, 10.11.2020 04:00

History, 10.11.2020 04:00

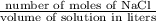 =
= 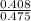 = 0.8589 M
= 0.8589 M

