
Chemistry, 20.07.2019 21:30 leeahnnfoster
Butane c4 h10 (-1.jpghf = –125.7), combusts in the presence of oxygen to form co2 (g) (mc016-2.jpghf = –393.5 kj/mol), and h2 o(g) (mc016-3.jpghf = –241.82) in the reaction: mc016-4.jpg what is the enthalpy of combustion, per mole, of butane?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Butane c4 h10 (-1.jpghf = –125.7), combusts in the presence of oxygen to form co2 (g) (mc016-2.jpghf...
Questions




Biology, 08.12.2020 02:20


Biology, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20




Mathematics, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20



Mathematics, 08.12.2020 02:20


Mathematics, 08.12.2020 02:20

Business, 08.12.2020 02:20

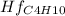 = -125.7 kJ/mol
= -125.7 kJ/mol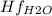 = -241.82 kJ/mol
= -241.82 kJ/mol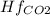 = -393.5 kJ/mol
= -393.5 kJ/mol

