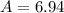Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of li-6 is 7.42% and the relative abundance of li-7 is 92.58%. based on this data alone, calculate the average atomic mass for lithium to the correct number of significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of...
Questions


Spanish, 05.07.2019 19:30





Computers and Technology, 05.07.2019 19:30

Mathematics, 05.07.2019 19:30



Physics, 05.07.2019 19:30


Mathematics, 05.07.2019 19:30


World Languages, 05.07.2019 19:30

History, 05.07.2019 19:30

History, 05.07.2019 19:30


Geography, 05.07.2019 19:30

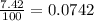
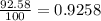
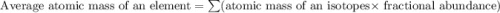
![A=\sum[(6.0151\times 0.0742)+(7.0160\times 0.9258)]](/tpl/images/0113/1342/3dcbf.png)