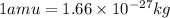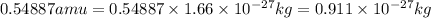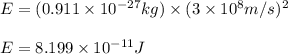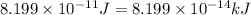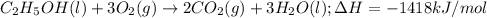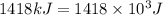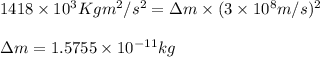3. when two atoms of 2h (deuterium) are fused to form one atom of 4he (helium), the total energy evolved is 3.83 × 10-12 joules. what is the total change in mass (in kilograms) for this reaction? 4. the mass of a proton is 1.00728 atomic mass units (amu) and the mass of a neutron is 60co nucleus whose nuclear mass is 1.00867 amu. what is the mass defect (in amu) of a 27 59.9338 amu? what is the mass defect in kilograms? what is the energy equivalent of this mass in kilojoules? 5. the equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into its equivalent mass. c2h5oh(l) + 3 o2(g) 2 co2(g) + 3 h2o (l) δh = -1418 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
3. when two atoms of 2h (deuterium) are fused to form one atom of 4he (helium), the total energy evo...
Questions

Biology, 18.11.2020 22:40





History, 18.11.2020 22:40



History, 18.11.2020 22:40




Arts, 18.11.2020 22:40




History, 18.11.2020 22:40

Spanish, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40

Health, 18.11.2020 22:40

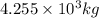
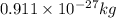 and energy equivalent to this mass is
and energy equivalent to this mass is 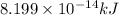
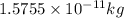

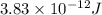
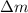 = mass change = ?
= mass change = ?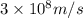
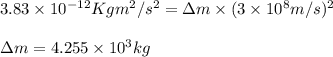
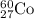
![\Delta m=[(n_p\times m_p)+(n_n\times m_n)+]-M](/tpl/images/0113/2964/f34f4.png)
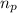 = number of protons = 27
= number of protons = 27
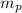 = mass of one proton = 1.00728 amu
= mass of one proton = 1.00728 amu
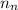 = number of neutrons = 33
= number of neutrons = 33
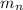 = mass of one neutron = 1.00867 amu
= mass of one neutron = 1.00867 amu
![\Delta m=[(27\times 1.00728)+(33\times 1.00867)]-[59.9338]\\\\\Delta m=0.54887amu](/tpl/images/0113/2964/c0b92.png)