
Chemistry, 20.07.2019 23:00 tashai3138
The acid dissociation constant for an acid dissolved in water is equal to the a. equilibrium constant b. equilibrium constant times the concentration of water c. equilibrium constant divided by the concentration of water d. equilibrium constant times the equilibrium constant of water

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3


Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
The acid dissociation constant for an acid dissolved in water is equal to the a. equilibrium consta...
Questions

Mathematics, 17.09.2019 03:00

English, 17.09.2019 03:00



History, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00




Biology, 17.09.2019 03:00


Social Studies, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00


Mathematics, 17.09.2019 03:00

Spanish, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00

Social Studies, 17.09.2019 03:00

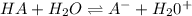
![K_{eq}=\frac{[A^-][H_3O^+]}{[HA][H_2O]}](/tpl/images/0113/3718/a0805.png)
![K_a=K_{eq}\times [H_2O]=\frac{[A^-][H_3O^+]}{[HA]}](/tpl/images/0113/3718/c2e7c.png)


