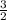Chemistry, 20.07.2019 23:30 jeffreyaxtell4542
One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, na3(co3)(hco3)·2h2o. 2na3(co3)(hco3)·2h2o(s) → 3na2co3(s) + co2( g. + 5h2o( g. when 1.00 metric ton (1.00 × 103 kg) of trona is decomposed, 0.650 metric ton of na2co3 is recovered. what is the percent yield of this reaction?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, na3(co...
Questions

English, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10


English, 18.10.2019 07:10

English, 18.10.2019 07:10



Biology, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

Mathematics, 18.10.2019 07:10

History, 18.10.2019 07:10

History, 18.10.2019 07:10


Biology, 18.10.2019 07:10

English, 18.10.2019 07:10